Chemistry
Thomson's atomic model
 |
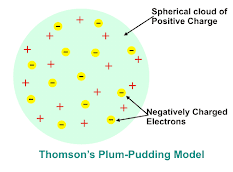
Thomson's Atomic Model
After the discovery of electron and proton J.J Thomson in 1898 tried to explain the arrangement of a electron and proton in an atom. He proposed that an atom is consist of a spear of positive electricity in which the electron are embedded like plum-pudding or seed evenly distributed in the red spongy mass of a watermelon .
👉 Thomson model of atom is also called as plum-pudding model because it just like raisins in the pudding.
👉The model able to explain some know text or properties of atom like electrically neutrality of atom.
👉It could not satisfy the experimental text and hence it was discarded .
Limitation
👉It could not explain the ionisation and scattering experiment performed by rutherford .
Previous article
This Is The Newest Post
Next article




Leave Comments
Post a Comment